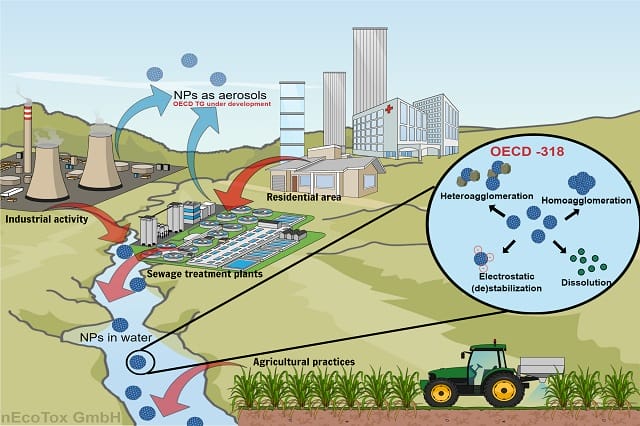
In a crude sense of matters, nanotechnology is all about Extremely small dimensions, one billionth of a meter, to be precise. At the nanoscale, the rules of the game are not the same as those we have been playing till now with respect to conventional chemicals and substances. On the one hand, nanotechnology has revolutionized advancements in numerous industries leading to large scale manufacture and consumerization of nanoforms. On the other hand, an increased use inevitably resulted in nanomaterials entering the environment via various routes as shown in the figure. Nanomaterials can be aerosolized during industrial practices, in wastewater treatment plants and even during natural processes, such as, volcanic eruption, leading to air pollution. Nanomaterials entering water, however, poses another concern as they can directly interact with aquatic life forms. What happens to nanomaterials in water? Do they dissolve generating ions? Perhaps, perhaps not. Are they mobile? Could be, could be not. Are they toxic? Possibly, possibly not.
In the late 2000s, nanotoxicology research opened a “black box”, which brought to light hitherto unknown effects of nanomaterials, some of which were detrimental to environmental and aquatic health, while others were largely benign. To completely assess nanomaterial behavior and toxicity in water, an array of information has to be evaluated simultaneously, such as,
– characteristics of the nanoparticle (NP) itself: morphology, size, surface functionality, surface area, density etc., and not only chemical composition, and
- Water quality: nature, chemistry and concentration of dissolved and particulate organic matter (DOM and POM), composition and concentration of ions, pH, temperature, salinity etc.
A few examples might be helpful to put this information into perspective. For example, spherical Ag-NPs behave differently than Ag nanorods in a typical freshwater environment. Titanium dioxide-NPs, Ag-NPs, ZnO-NPs, and silica-NPs, all of the same size and shape, may exhibit different dispersion or aggregation dynamics in water. The change of water matrix from freshwater to saline or brackish water also affects the behavior of nanomaterials. Although this behavior of nanomaterials may seem frustrating from a regulatory perspective, oddly enough, these different responses are largely due to a single fundamental property of the nanomaterial: dispersion stability. In water, NPs can interact with themselves (homoagglomeration), with DOM or POM in water (heteroagglomeration), with anions and cations in water (electrostatic stabilization or destabilization depending on NP surface charge) or, they can dissolve, resulting in the leaching of ions into the water. Quantification of dispersion stability is key information to adequately assess mobility, settling (on soil or in sludge), and bioavailability and/or bioaccumulation (toxicity), ultimately enabling sound hazard and risk assessment of nanomaterials in water.
The new OECD guideline No. 318 , is the first standardized test guideline (TG) for quantifying the dispersion stability of nanomaterials in the aquatic environment. In a typical test system, a freshwater environment is simulated with DOM, electrolyte, and pH conditions observed in 95% of freshwater sources, and the dispersion stability of nanomaterials is studied over a period of time. To compensate for the differences arising from the specific physicochemical properties of nanomaterials, the guide offers a simple solution: to consider the "number concentration" of nanomaterials instead of the commonly used "mass concentration". Determining the number concentration requires environmentally relevant physicochemical data that define the nanomaterial, such as the shape, size, surface area, and density of the particles, whereas determining the mass concentration requires only the chemical formula of the substance. This approach ensures the inclusion of nanomaterial properties and water quality parameters in a single testing protocol. The results of this test classify nanomaterials as primarily very stable, least stable, and intermediate stable in the aquatic environment, which can be further used for risk assessment.
We, the nEcoTox GmbH Perform stability analysis for nanoparticle dispersions according to OECD TG-318. OECD GD-318 also includes information on quantifying the dissolution of nanomaterials in water, a service we also offer. We can also assist you in characterizing your nanoforms by providing the information required for REACH registration.
To learn more about how we conduct these tests at our facility, join the short but informative online Q&A webinar hosted by Dr. Ricki Rosenfeldt on “Dispersion Stability of Nanomaterials – OECD TG-318” on 16th February 2021 at 10 am (CET). Jetzt anmelden here.
For more information please contact me by phone: 06346/9661490 or by e-mail: menon@necotox.de